Assalam-o-Alaikum Dear!
What is Surface Tension and explain its properties?

Today we discuss surface tension. Surface tension is the property of the liquid surface that is displayed by its acting as if it were stretched elastic membrane. Surface tension is the property of liquid to shrink it into a possible minimum area. Liquids have cohesion and adhesion, both of which are forms of molecular attraction.

Cohesion:
Cohesion enables a liquid to resist tensile stress.
Adhesion:
Adhesion enables it to adhere to another body.
Explanation:
At the interface between a liquid and gas i.e. at the liquid surface and at the interface between two immiscible liquids,(immiscible means not mixable.) the out-of-balance attraction force between molecules forms an imaginary surface film that exerts a tension force in the surface. The liquid property is known as surface tension. Because this tension acts on a surface, we compare such forces by measuring the tension force per unit length of the surface. When a second fluid is not specified at the interface, it is understood that the liquid surface is in contact with air. The surface tension of various liquids covers a wide range, and they decrease slightly with increasing temperature. In the comparisons, organic liquids such as benzene and alcohol have low surface tension. And the mercury has larger surface tension. As the increase of temperature then the forces of molecular attraction are less so surface tension also decreases.

The surface tension values for the liquid-like water between the freezing points( 0.0756 N/m) and boiling points (0.0589 N/m) are different.

Examples:
This phenomenon of surface tension can be observed in the approximately spherical shape of water drop and bubble of soap. Certain insects can stand on the surface of the water.
A razor blade can also support this process of surface tension. if we pushed the razor in the water then it cannot be floated in it, it sinks through the surface of the water.
Surface tension means the number of forces exerted on the surface perpendicular to the line of unit length.

SI Unit:
The SI Unit is newton per meter which is equivalent to the joule per square meter.
Dimension:
The dimension of the surface tension is [M T^-2].
Dependence:
The surface tension mainly depends upon the forces of attraction between the molecules in the given liquid and upon the gasses, solid or liquid in contact with it.

Properties of Surface Tension:
There are different properties of surface tension.
Capillarity:
Capillarity is the property of the forces exerted on a fluid by fine tube or porous media; it is due to both cohesion and adhesion. When the cohesion is of less effect than the adhesion, then the liquid will wet a solid surface it touches and rises at the point of the contact; if cohesion predominates, the liquid surface will depress at the point of the contact.
For example:
Capillarity makes the water level rise in a glass tube and the mercury depress below the true level that is drawn into the scale and reproduced the actual size. We called the curve liquid surface that develops in a tube called the meniscus.
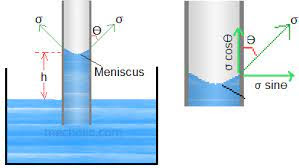
Mathematical Form:
A cross-section through a capillary rise in a tube body.From free body considerations, equating the lifting force created by the surface tension to the gravity force,
2𝜋𝑟𝜎𝑐𝑜𝑠𝜃 = 𝜋𝑟^2ℎ𝛾
ℎ = 2𝜎𝑐𝑜𝑠𝜃/r
γ
Where 𝜎 = that is for surface tension (sigma ) in units of force per unit length
𝜃 = that is for wetting angle (theta) 𝛾 = that is for the specific weight of liquid r = that is for the radius of tube h = that is for capillary rise
We can use this expression to compute the approximate capillary rise or depression in a tube. If a tube is clean then 𝜃 = zero degrees for water and 140 degrees for mercury. The meniscus lifts a small volume of liquid, near the tube walls, in addition to the volume 𝜋r^2h used. For large tube diameters, with smaller capillary rise heights, this small additional volume can become a large fraction of 𝜋r^2 h. The curves are for water or mercury in contact with air; if mercury is in contact with water, the surface tension effect is slightly less than when in contact with air. For tube diameters larger than in (12mm), capillary effects are negligible.







0 Comments